 Aspirin, or 2-acetobenzoic acid is on the list of the World Health Organisation's list of essential medicines. It has been available in pills for over one hundred years (1899 to be precise). Most people have taken aspirin for pain relief and more recently as an anti-inflammatory. In addition, there are some much more specific illnesses that are treated with aspirin. The Bayer company were the first to synthesise the drug and copyright the name aspirin, but its therapeutic power has been known since the time of the Ancient Egyptians and later Hippocrates the Greek physician (around 400BC) wrote that willow leaves and bark brought pain relief and eased fever. We now know that the active agent is salycilic acid, which in commercial aspirin has the 2-OH group acetylated. The success of aspirin made Bayer a very wealthy pharmaceutical company. Eventually however, the introduction of paracetamol and ibuprofen led to a significant drop in the demand for aspirin. However the discovery by Sir John Vane that aspirin exhibits anti blood clotting activity, has subsequently seen a resurgence in the use of aspirin.
Aspirin, or 2-acetobenzoic acid is on the list of the World Health Organisation's list of essential medicines. It has been available in pills for over one hundred years (1899 to be precise). Most people have taken aspirin for pain relief and more recently as an anti-inflammatory. In addition, there are some much more specific illnesses that are treated with aspirin. The Bayer company were the first to synthesise the drug and copyright the name aspirin, but its therapeutic power has been known since the time of the Ancient Egyptians and later Hippocrates the Greek physician (around 400BC) wrote that willow leaves and bark brought pain relief and eased fever. We now know that the active agent is salycilic acid, which in commercial aspirin has the 2-OH group acetylated. The success of aspirin made Bayer a very wealthy pharmaceutical company. Eventually however, the introduction of paracetamol and ibuprofen led to a significant drop in the demand for aspirin. However the discovery by Sir John Vane that aspirin exhibits anti blood clotting activity, has subsequently seen a resurgence in the use of aspirin. |
| Aspirin tablets then and now! |
The synthesis of aspirin is such a nice example of simple preparative organic chemistry, that it is widely used to illustrate the principles of reaction, isolation, separation and crystallisation. It serves as an excellent test of laboratory skills, in particular in respect of yield, purity and analysis. The reaction used in many lab classes is shown below. A good source of information for molecules of this type is PubChem: you can find the link to aspirin here.
The standard protocol is as given in detail below, but let's look at some of the steps and reagents (highlighted). Acetic anhydride (AcA) is a popular and effective acetylating agent. Structurally, AcA is flexible in solution and the negative dipoles on the carbonyl oxygens tend to destabilise AcA and promote its reactivity. The carbonyl carbon provides an electrophilic character and the carboxylate moiety is the leaving group: this internal asymmetry is at the heart of the reactivity of AcA. The mechanism of the reaction is shown below for those interested
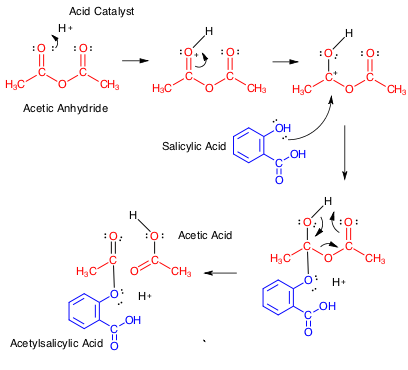
The reaction proceeds more effectively under acid conditions: the sulphuric acid is acting as a catalyst in some respects. Crystallisation in organic chemistry (and to a certain extent in biochemistry) is used to remove contaminants that may show subtly different levels of solubility in the crystallisation solvent and it also serves to concentrate the product and in industry, processing and downstream dissolution of solids can be controlled more easily.
Finally the important concept in determining yield in organic synthesis is to ensure that the product that is weighed, contains no superfluous solvent (often water): this must be driven off in an oven, before a robust calculation of yield can be made. One point to note is that the compound being synthesised MUST be stable under the drying conditions, or must show no tendency to change its racemic state.
- Place 2.0 g (0.015 mole) of salicylic acid in a 125-mL Erlenmeyer flask.
- Add 5 mL (0.05 mole) of acetic anhydride, followed by 5 drops of conc. H2SO4 (use a dropper, H2SO4 is highly corrosive) and swirl the flask gently until the salicylic acid dissolves.
- Heat the flask gently on the steam bath for at least 10 minutes.
- Allow the flask to cool to room temperature. If acetylsalicylic acid does not begin to crystallize out, scratch the walls of the flask with a glass rod. Cool the mixture slightly in an ice bath until crystallization is completed. The product will appear as a solid mass when crystallization is completed.
- Add 50 mL of water and cool the mixture in an ice bath. Do not add the water until crystal formation is complete.
- Vacuum filter the product using a Buchner funnel. You can use some of the filtrate to rinse the Erlenmeyer flask if necessary.
- Rinse the crystals several times with small portions (5 mL) of cold water and air dry the crystals on a Buchner funnel by suction until the crystals appear to be free of solvent.
- Record the weight of the crude solid which probably contains water.
- When the product is completely dry, weigh the product, determine its melting point (lit mp 135-136 °C) and calculate the percentage yield.
The mode of action of aspirin remains inconclusive and involves interactions with the enzymes of prostaglandin biosynthesis, cyclo-oxygenases, which you can read about here. The uncertainty of aspirin's mode of action relates mainly to its pain relief (analgaesic) effect and this perhaps reflects our relatively poor understanding of pain reception in general.

No comments:
Post a Comment